Description
What is a molecule?
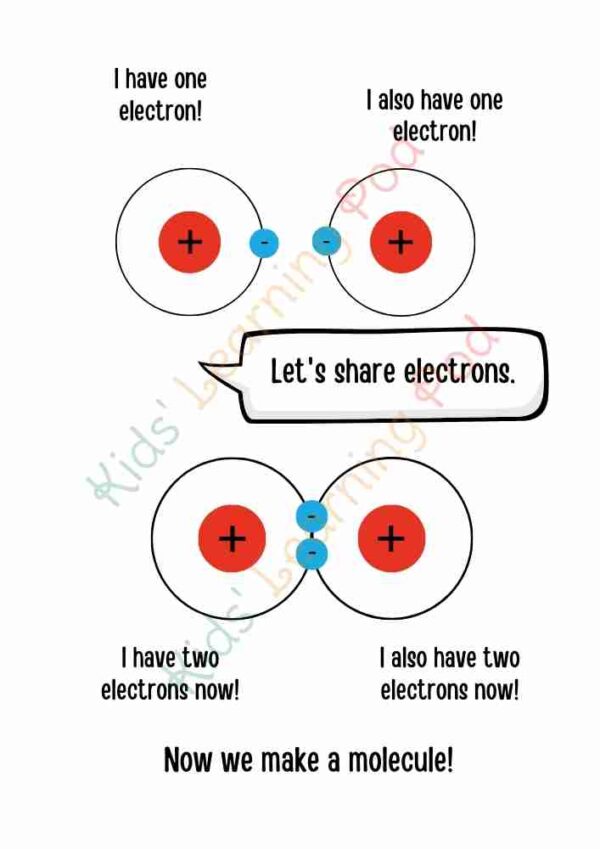
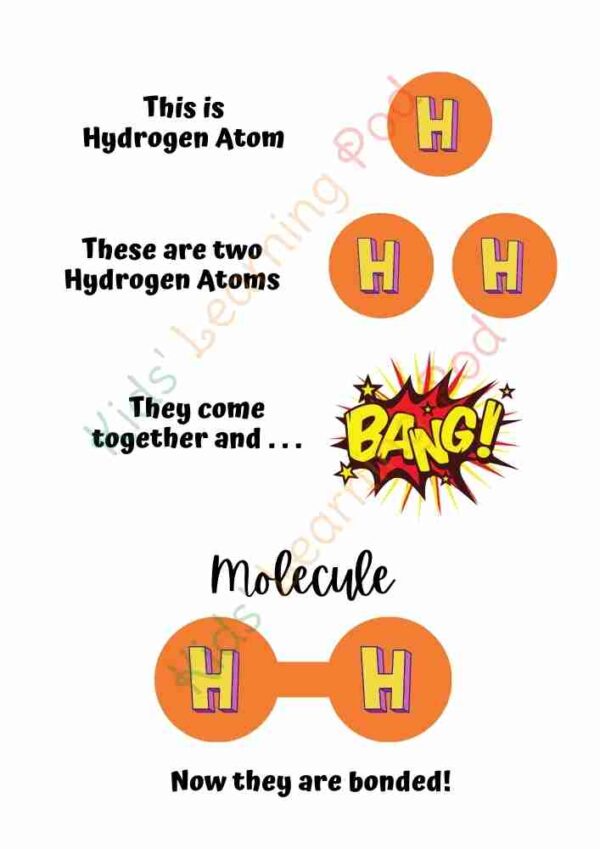
A molecule is a group of atoms that are chemically bonded together. These atoms can be the same or different, and they can be bonded together in various ways to form different types of molecules. Molecules make up all matter, including gases, liquids, and solids. They are the building blocks of matter and are responsible for the properties and behavior of different types of materials. Some examples of molecules include water (H2O), oxygen (O2), and carbon dioxide (CO2). The study of molecules and their properties is called chemistry.
Teaching toddlers about molecules can be a challenging task, as they are still developing their language skills and understanding of complex concepts. Here are a few tips on how to introduce the concept of molecules to toddlers:
Here are a few examples to understand what is molecule:
Water molecule (H2O)
Water is a liquid that is essential for life and is found all around us. It is made up of two hydrogen atoms and one oxygen atom.
Oxygen molecule (O2)
Oxygen is a gas that we need to breathe in order to survive. It is made up of two oxygen atoms.
Carbon dioxide molecule (CO2)
Carbon dioxide is a gas that is produced when we breathe out and when certain things burn. It is made up of one carbon atom and two oxygen atoms.
Salt molecule (NaCl)
Salt is a solid that is used to add flavor to food. It is made up of one sodium atom and one chlorine atom.
Sugar molecule (C12H22O11)
Sugar is a sweet substance that is found in many foods. It is made up of 12 carbon atoms, 22 hydrogen atoms and 11 oxygen atoms.
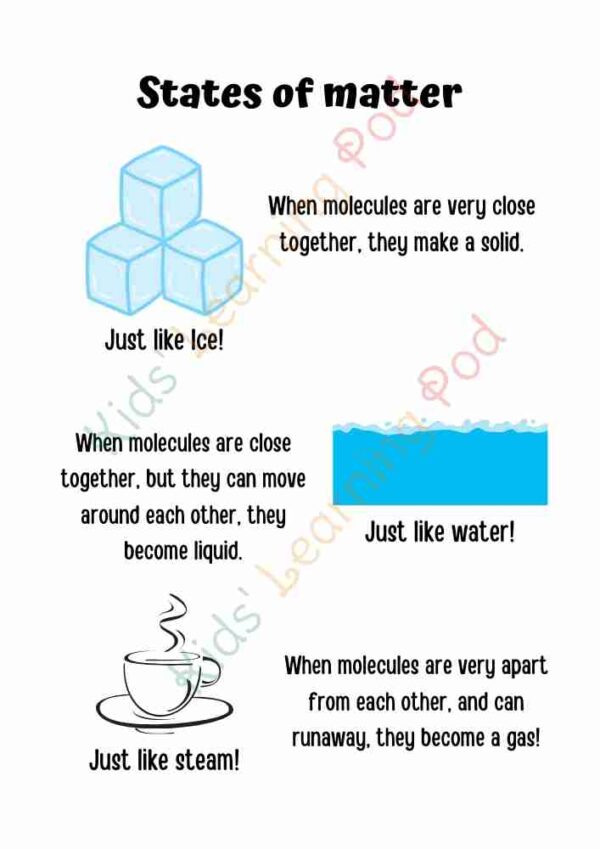
Ice molecule (H2O)
Ice is a solid form of water, and it is made up of the same molecules as liquid water.
It’s important to note that the above example are simplified versions of the real molecule and the structure of the molecules can be quite complex, but these examples can help children understand the concept of molecules that exist around them and how they are formed.
What is the shape of a water molecule?
The shape of a water molecule is a bent or angular shape, also known as a “V-shape” or “tetrahedral” shape. This is due to the fact that the two hydrogen atoms are located at a 105-degree angle to the oxygen atom in the center. The reason for this shape is due to the arrangement of the electrons in the molecule. The oxygen atom has a higher electronegativity, which means it attracts electrons more strongly than the hydrogen atoms. This results in a polar covalent bond, where the electrons are not shared equally between the atoms. The oxygen atom has a partial negative charge, while the hydrogen atoms have partial positive charges. This creates a dipole moment, which gives the water molecule its bent shape.
Want to read more science books?
Let your kids explore more about science, read “Human Cell”. Follow the link to read this book.











Reviews
There are no reviews yet.